The Limits of Animal Photosynthesis
Some back-of-the-envelope estimates on chloroplasts and mammalian energy.
This article was written by Sam Clamons. It is entirely his own work, and it is a bit speculative, but I really enjoyed reading it and am excited to share it with you!
In 2014, biologists from Harvard University inserted living cyanobacteria into developing zebrafish. The cyanobacteria continued to live and grow in their animal hosts for at least 12 days without disrupting the fishes’ development.
Just last year, researchers at the University of Japan confirmed that a protistan red algae, once inserted into mammalian cells, continued to photosynthesize for at least two days.
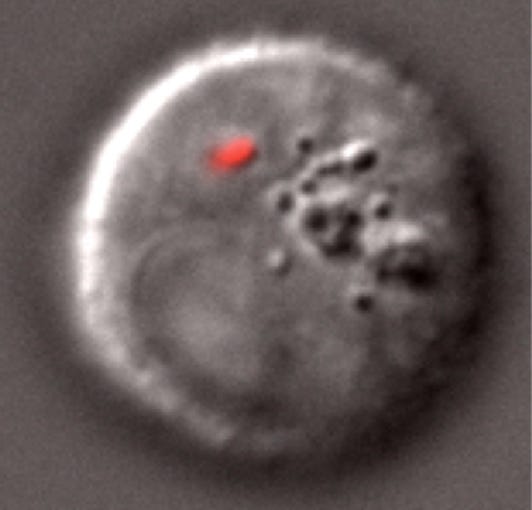
These kinds of synthetic biology experiments typically serve the dual purposes of answering key biological questions and expanding our engineering capabilities. Sticking photosynthetic organisms into animals helps us understand how the ancient ancestors of today’s eukaryotes once incorporated foreign bacteria to create new organelles. By “trying out” some of the answers evolutionary biologists have hypothesized, synthetic biologists can learn which paths are easier or harder to achieve, and thereby which theories are more likely or less likely.
On the flip side, being able to slot new bacteria into eukaryotic cells like USB devices on a laptop would unlock a huge range of new capabilities, just as the incorporation of mitochondria and chloroplasts in nature led to an explosion of evolutionary diversity.
The idea of enhancing animal cells with functioning chloroplasts also leads naturally to a more theoretical, playful question—could it be possible to engineer a human or a mouse to get all of their energy from photosynthesis, through some novel symbiosis with chloroplasts or cyanobacteria?
Unfortunately, the answer is no. Even ignoring the nutrients that photosynthesis can’t provide—like salts, trace minerals, vitamins, and amino acids—photosynthesis simply can’t provide enough power to keep a human or a mouse going. To understand why, we’ll need to look at the theoretical power human photosynthesis could provide, and compare it to the typical power consumption of a human.
It’s convenient to think about human power consumption in units of energy burned per second, typically measured in joules per second, or watts (W). How many watts a human needs depend on their metabolism, size, and level of physical activity. Most people burn about 1 watt per kilogram of body weight during normal, non-strenuous activity. Assuming a hypothetical person who weighs 100 kilograms, that comes to about 100W (or, in more common dietary units, almost exactly 2000 calories per day).
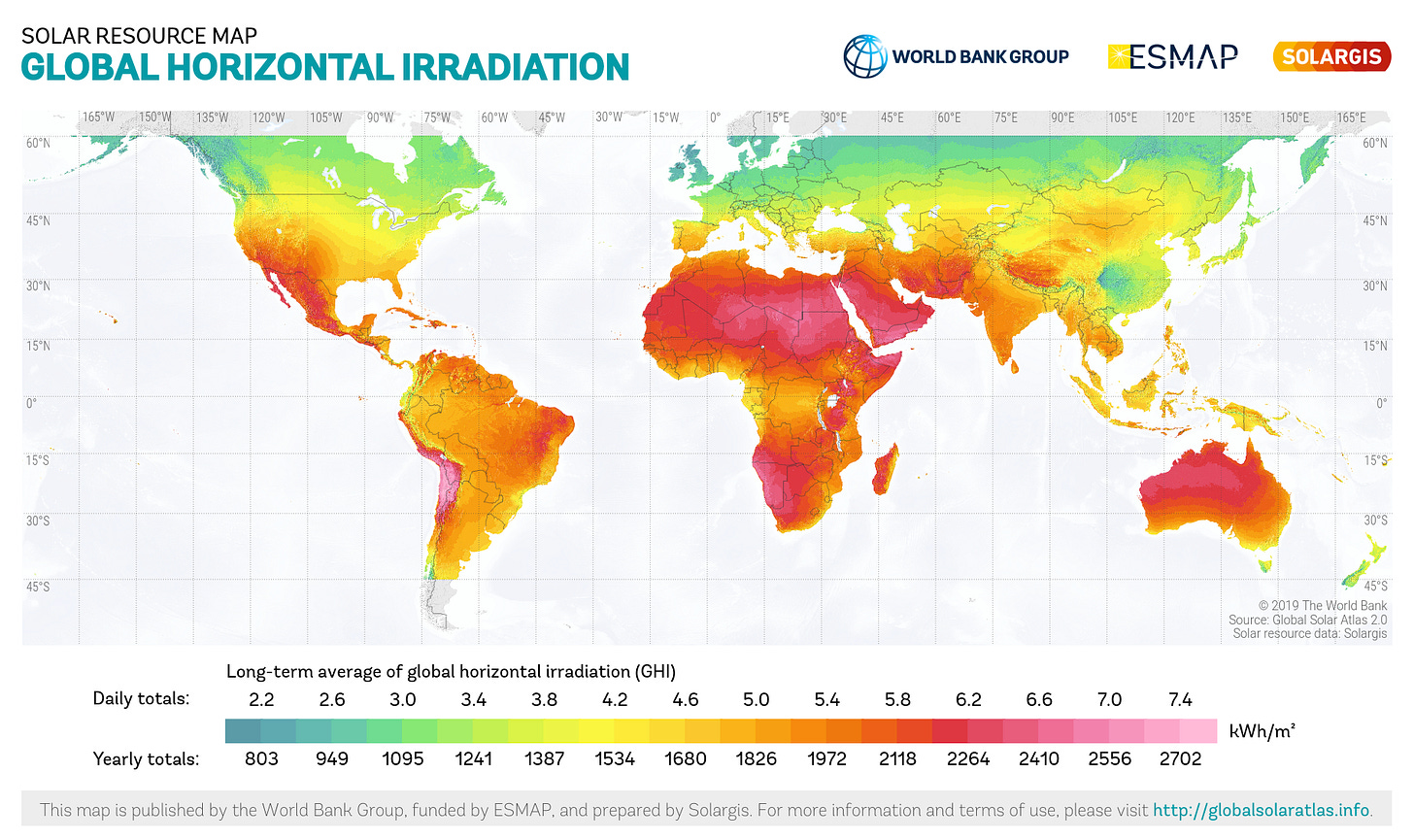
No matter how well-optimized, no photosynthetic system—natural or otherwise—can produce more energy than it receives from the sun. Just how much light the surface of the Earth receives varies from place to place and can be measured a number of ways, but the most relevant here is long-term averaged global horizontal irradiance (GHI).
GHI is the total amount of light energy (both directly from the sun and scattered off the atmosphere) hitting some region per unit area, accounting for latitude, altitude, atmospheric absorption and reflection (including cloud cover), and daylight length, averaged over a typical year. Here are some handy GHI values for reference, converted to units of watts per square meter:
Low light: 100 W/m2 (Northwest Canada, Cambridge UK, lowland New Zealand)
Medium light: 210 W/m2 (Central California, southern Spain, most of India)
Maximum light: 310 W/m2 (Northern Australia, central Andes, Sahara desert)
How much sunlight a photosynthetic skin can harvest depends on how much of that skin is actually exposed. A roughly-average human possesses 1.7 m2 of skin, but typical American-style summer clothes (leaving arms, head, most of the legs, and some of the upper chest exposed) only leave about 60% of that skin exposed. Winter clothes, covering everything except hands and head, cut that down to 10%.
Finally, photosynthesis does not, and cannot, harvest light with perfect efficiency. Every step of the process bleeds energy as waste heat or light.
To begin with, the pigments used by plants can only capture light in a narrow range of wavelengths, which makes about half of sunlight energy totally useless. Even in that range, more energetic photons carry more energy than the photosystem complex can usefully absorb in one go. Excess is unavoidably lost, eating up another 15% or so of remaining energy. Most of the rest is lost somewhere in the long chain of chemical reactions that convert the captured light into stored sugars. One careful accounting puts an upper limit on the theoretically possible photosynthetic efficiency at 6% of incoming light energy; that same source finds that real-world crop plants, which are highly optimized for efficiency, top out at around 4%.
Getting chloroplasts in human skin to even match the efficiency of plant chloroplasts would be a herculean achievement. Chemistry aside, plants have some astounding and intricate physiological adaptations to make photosynthesis more efficient. They can breathe directly through the skin of their leaves. Leaves are optical light traps, holding unabsorbed photons longer than their bulk physical properties would suggest they should. The most efficient photosynthesizers (C4 plants) have specialized, compartmentalized tissues for different steps of photosynthesis. All of these adaptations would, somehow, have to operate alongside the mammalian physiology required for sweating, growing hair, replacing skin, and other functions. And the chloroplasts themselves don’t come free—chloroplasts can take up fully half of the volume of some photosynthetic organisms!
For the sake of optimism (and tractability), let’s assume we can someday get human or mouse skin to match leaves in terms of photosynthetic yields. Then, using the 4% peak efficiency of real-world C4 crop plants as a proxy for hypothetical engineered-skin photosynthetic efficiency, we can calculate the fraction of a 100W energy budget that could be supplied with a range of skin exposure and locales:
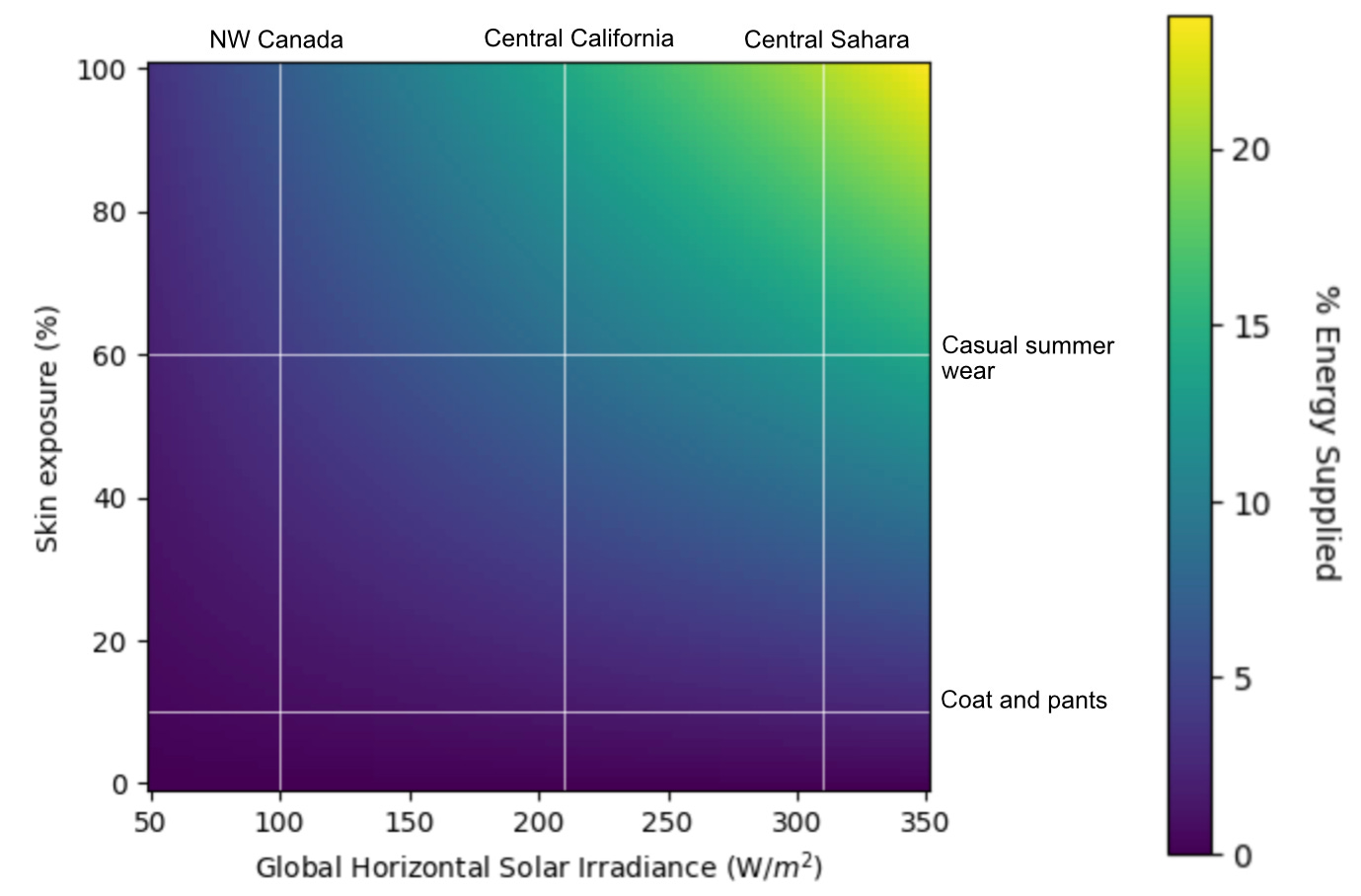
At the most optimistic, a chloroplast-armed human with enough chloroplasts and other physiological adaptations to match the highest practical efficiencies of plants, living naked in the Sahara and running at 100W (2000 kCal/day), could get about a quarter of their caloric needs from sunlight. Moving to the Northwest Territory and bundling up for winter would cut that fraction down to 2.5%.
You, dear reader, are unlikely to be a perfectly average human. If you are bigger or smaller than 100 kilograms, you’ll need proportionally more or less energy—but you’ll also have more or less skin to collect sunlight with! The two values—mass and surface area—don’t scale at the same rates, though. Surface area scales with the square of height; mass with the cube of height. Therefore, a smaller human could get more of their energy budget from the sun than a larger human could.
Unfortunately, even smaller mammals could only get a small fraction of their total energy needs from photosynthesis. This is partly because, although smaller mammals have much larger surface area per unit volume than larger ones, they also burn hotter. Various researchers have calculated different relationships between volume and base metabolic rate, but a reasonable estimate is that total energy burn scales roughly with mass to the ¾th power. A large house cat has only 5% the mass of our “typical” human, but requires more than 10% of that same human’s daily calories. By the same formula, we can expect an Asian elephant, with a typical mass of about 40 people, to only eat as much as 403/4 ~ 16 people (calorically speaking).
Combining the above metabolic scaling with surface area and volume scaling, we can estimate the benefits of photosynthesis for a range of mammals of different sizes:
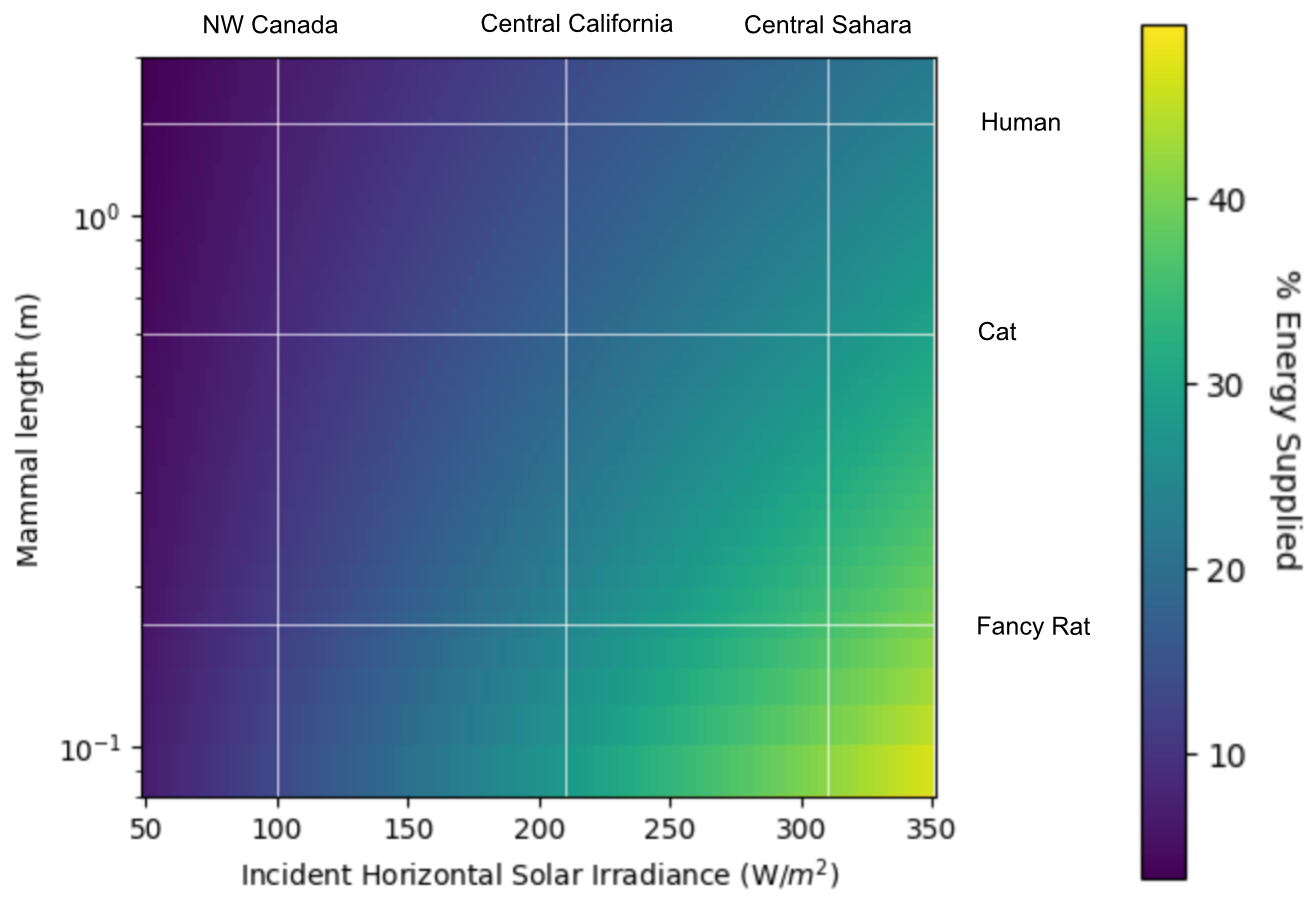
Extrapolating this chart down a bit, we find that photosynthesis could, theoretically, completely meet the caloric needs of a mammal living in the Sahara… if that mammal were five millimeters long.
In conclusion, introducing photosynthetic organelles and organisms to animals can inform our understanding of the deep history of eukaryotic evolution, and may pave the way for powerful medical techniques using engineered endosymbionts—but we shouldn’t hold out any hope for living off of sunlight and vitamin supplements alone.
Even in the best-case conditions, photosynthesis on Earth could only provide a small fraction of the energy needs of any mammal of reasonable size (humans included). It seems that the mammalian lifestyle is simply too energy-intensive for autotrophy alone.
Samuel Clamons is a bioinformatics scientist at Illumina, Inc. with a PhD in Bioengineering and training in applied mathematics and computer science. Outside of his day job, he writes science fiction and researches theoretical questions in biology at Asimov Press.
